INTRODUCTION
Chicken eggs are a predominant complete food which has also the benefit of being inexpensive. They are also rich in vitamins (vitamin A, vitamin B6, vitamin D), minerals (selenium) fatty acid (oleic acid, linolenic acid) and proteins (ovalbumin, ovotransferrin, ovomucin) [1]. Due to their multifunctional properties such as foaming, gelling, coagulation and emulsifying they represent an important segment of the world food industry [2]. Global total egg production in 2018 was 76.8 million tons [3] and local total table egg production was 173.7 million in 2019 [4]. However, eggs are highly susceptible to internal quality deterioration and microbial contamination during storage. These conditions can cause serious economic losses to the poultry industry [5].
During storage, higher pH and thin albumen is caused due to quality changes in albumen and yolk by the fact that moisture and CO2 is loss via the shell pores [6]. Chemical, physical, microbial, and functional properties of eggs start to change as soon as they are laid therefore with the increase of storage time microbial contamination and the susceptibility to internal quality deterioration increase [7]. Shell eggs having a short shelf life (7–10 days) at room temperature. Therefore, preserving eggs becomes important to extend the shelf life of eggs. Among the methods used, low temperature refrigeration is recommended where the shelf life will be extended to five weeks [8]. But, certain barriers such as refrigeration not been available in rural areas and high cost for refrigeration have shown that refrigeration is not a convenient solution for all [9]. As an alternative method, surface coatings can be used to extend shelf life and thus minimize economic loss. In addition to that surface coating on eggs can increase shell strength thus reducing number of cracked eggs [10].
In food industry, edible films and coating play an important role due to its versatile properties such as providing semi-permeable barrier against gases and moisture, reducing respiration, water loss and oxidation reaction rate. Synthetic polymers, polysaccharides and proteins are applied as various coating materials to maintain the shelf life [11]. Currently, mineral oil is used as a coating material. But several problems are associated with the use of mineral oil which include, mineral oil being a petroleum-based product and taking a long time for drying [12]. Therefore, attention has been moved on the use of waxes as coating materials. Waxes are most commonly used hydrophobic film forming barrier material [13]. Different types of waxes such as beeswax, shellac wax, paraffin wax, carnauba wax and candelilla wax are being used. Edible or wax coatings are applied by dipping, brushing, or spraying wax onto the surface of the product. After waxing, a thin layer of wax adheres tightly to the surface, reducing the respiration rate and sealing the moisture of products [14]. Among them, plant waxes are found in common, cheap in price, light in color, non-toxic and biodegradable.
“Boomi” (Litsea glutinosa) is found throughout Sri Lanka. Due to its ayurvedic value, it is used for treatments of diarrhea, dysentery, sprains, bruises, boils and rheumatism [15]. It showed high antibacterial effect Bacillus subtilis, Escherichia coli, and Staphylococcus aureus. The bark mucilage consists of hetero-polysaccharide polyuronides consisting of sugar and uronic acid units which are formed from the cell wall and deposited on it in layers. These polyuronides swell in water and form a gel which is then used as binding agent. Experimentally proven to be a powerful binding agent, the bark mucilage is in formulations for tablets [15].
“Dawul kurundu” (Neolitsea cassia) is a plant native to Sri Lanka. Its leaves are main mucilaginous materials used in traditional culinary practices and it showed characteristic cinnamon odor [16]. “Dawul kurundu” had a good swelling index (27.8%). This mucilaginous material had acidic nature and had acceptable organoleptic properties and micrometric properties. In the method of toddy tapping, the powdered leaf is used to the cut surface of coconut inflorescences [17]. Due to its ayurvedic value, it is used for treatments of fractures and skin rash. It also used for domestic food applications and it is consisted with antioxidant, antibacterial, and anti-inflammatory properties [16]. The weight loss was reduced in great extent by the “dawul kurundu” coating since this micro film act as an additional layer over the leaf surface while covering the stomata holes [18]. There is no information available on the effect of “boomi” tree wax and “dawul kurundu” wax on internal quality and shelf life of eggs during storage. So, the objective was to check the suitability of these two plant waxes as external coating materials on the internal qualities and shelf life of chicken eggs stored at room temperature.
METHODOLOGY
“Boomi” (Litsea glutinosa) wax and “dawul kurundu” (Neolitsea cassia) wax were collected from Teldeniya, Kandy area. Wax solutions were prepared on the day of coating experiment. Unwashed, Clean, brown shell, medium size (55–60 g) 300 eggs were purchased from layer farm at Demodara area. Coating materials were applied within 24 hours of laying.
Pre-trials were conducted to select the best solution from 1:4, 1:6.5, 1:9, and 1:11.5 combination (weight basis) by preparing “Boomi” Wax (BW): distilled water (DW) and 1:5, 1:7.5, 1:10, and 1:12.5 combination (weight basis) by preparing “dawul kurundu” Wax (KW): DW as the coating treatments. “Boomi” wax and “dawul kurundu” wax was weighed with an electric balance (Model: WT200001X, WANT Balance Instrument, Paraguachi, Venezuela). “Boomi” wax was centrifuged (Model: SORV ST 40R) at 1,295 ×g for 20 min and supernatant was separated. Best dilution ratio was selected by observing the thickness of the solution and spreading ability on the egg shell.
Eggs were individually weighed before coating and after coating with a balance. The eggs were coated with mineral oil, “boomi” wax and “dawul kurundu” wax by dipping. Four coating treatments were evaluated throughout the storage period. Negative control (NC) non-coated eggs, positive control (PC)- mineral oil coated eggs (MO), “boomi” wax coated eggs, “dawul kurundu” wax coated eggs. All eggs were placed in a narrow end down position in plastic egg trays and stored at room temperature (27°C) for 6 weeks. Each egg was considered as one replicate and 72 eggs were coated with same treatment.
Weight loss of the whole egg during storage was calculated as with the following equation [19].
All the weight was measured in grams. The weight of whole egg was measured with a balance. Three measurements for each treatment taken in weeks intervals.
Yolk color, Haugh unit (HU) and egg quality was measured by egg analyzer (Model: SN: EA1535 2015). Three measurements per treatment was taken weekly. The yolk width was measured by digital vernier caliper and yolk height was measured by hougher meter. Each value was taken with averaging three different points of yolk. The yolk index was calculated as yolk height/yolk width. Three replicates per treatment was taken weekly.
Albumen and yolk were separated by using egg separator. pH of the albumin and yolk were obtained suing a pre calibrated digital pH meter (Model: PL 700 pv). Three replicates per treatment was taken weekly basis. Air sac of eggs was identified using an Egg Candler. Then height of the air sac was measured using a Venire caliper. Three measurements per treatment was taken weekly basis.
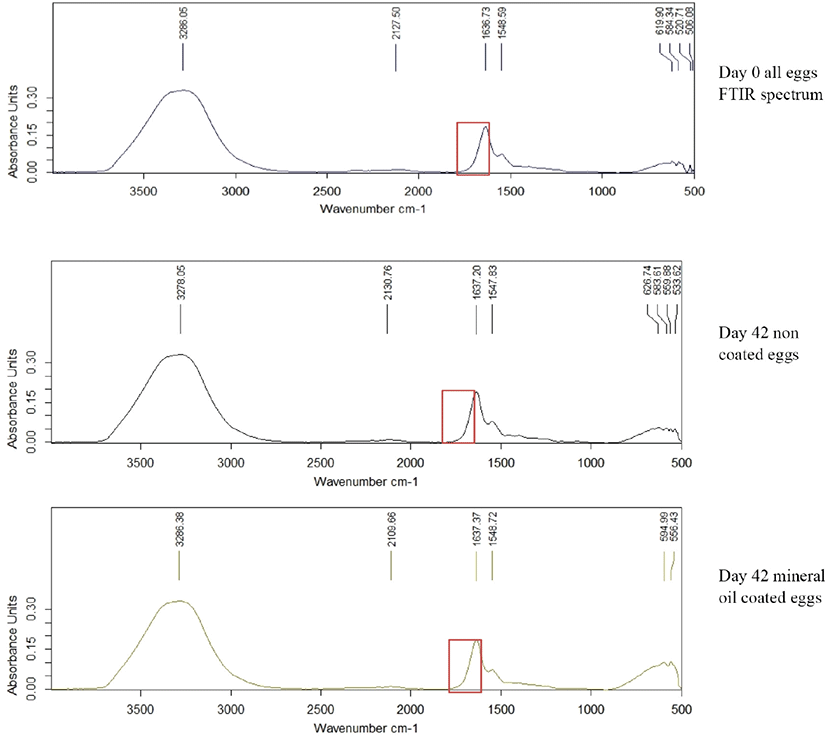
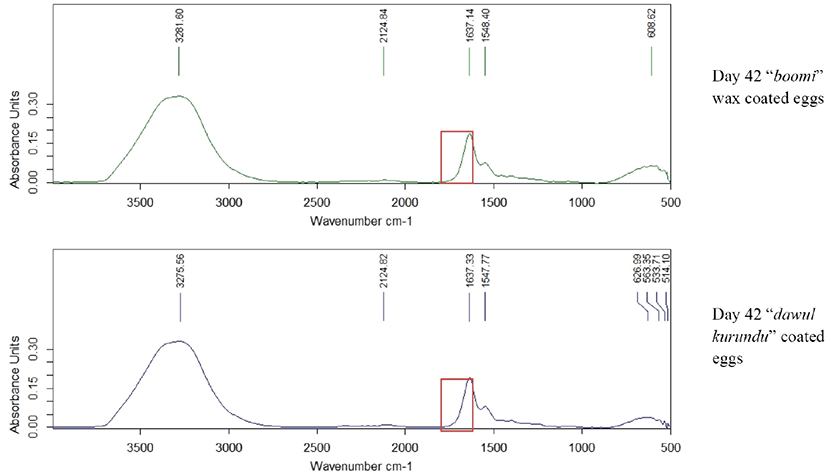
Fourier Transform Infrared single diamond ATR analysis was done for egg albumen and yolk of all treatments in weekly interval for 6 weeks of storage. Egg albumen and yolk was mixed for 4 min in a clean beaker using magnet stirrer. A drop of albumen and yolk was placed onto the surface of the ATR diamond crystal and allowed to air dry. Spectra were recorded with a resolution of 2 cm–1, and 32 scans were averaged for each spectrum (scan 4,000–650 cm–1). Difference in absorbance unit with the wave number was measured.
All the data were expressed as means with standard deviation with three replicates. Difference between mean values of three replicates groups were analyzed by one-way analysis of variance (ANOVA) and sensory data were analyzed using Friedman test. Statistical difference was considered at p < 0.05. Data were analyzing using MINITAB statistical software package and Microsoft office excel software package.
RESULTS AND DISCUSSION
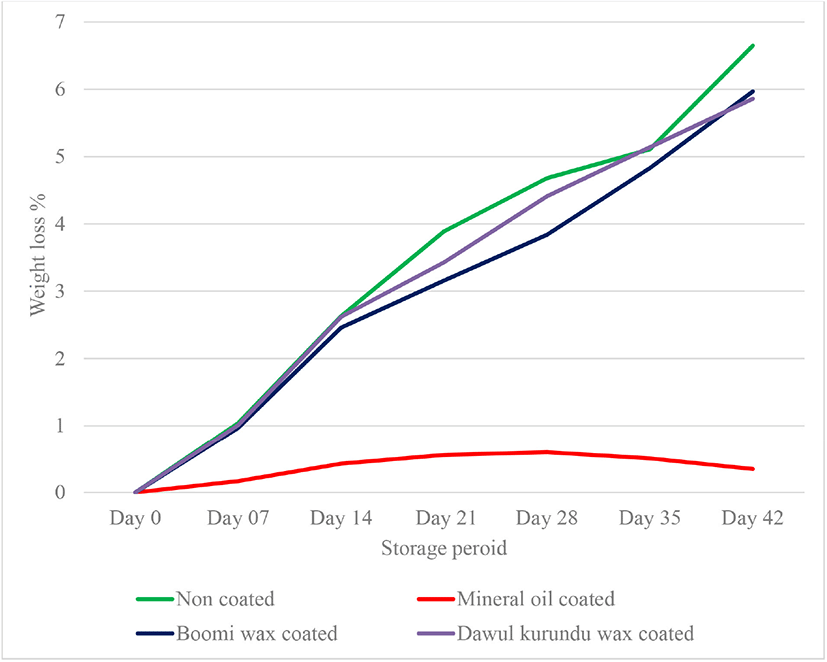
This is one of the important measurements to monitor the changes in quality of fresh shell eggs during storage. Overall weight loss gradually increased with increased storage periods. Due to the loss of water and CO2 from the egg albumen through the shell course the overall weight loss of the whole egg [20]. Eggs coated with mineral oil, “boomi” wax and “dawul kurundu” wax had significantly lesser weight loss than non-coated eggs throughout 6 weeks of storage periods (p < 0.05; Fig. 1). This study demonstrated that mineral oil and “boomi” wax coatings can offer a protective barrier against the loss of moisture through eggshell, thus reduce weight loss.
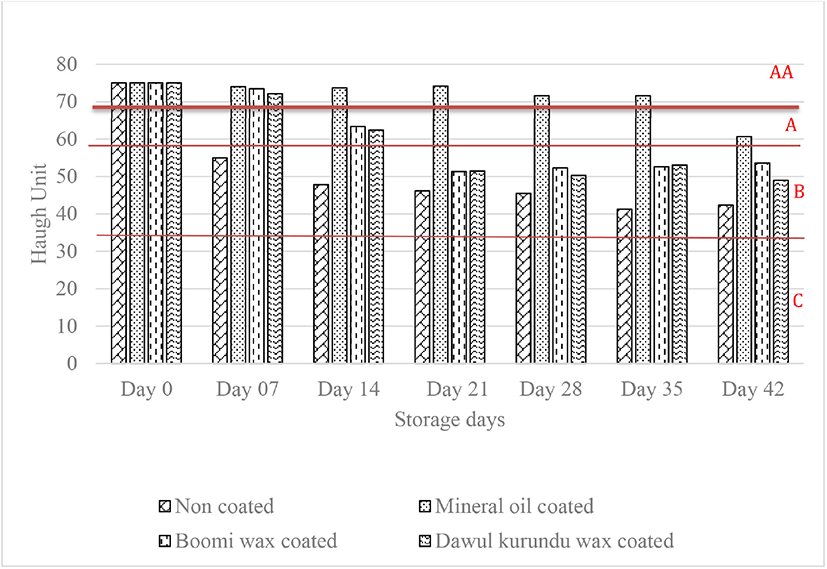
The higher the HU value, the better the albumen quality of eggs [7]. Changes in the HU (used for measurement of the albumen quality) of non-coated and coated eggs are shown in Fig. 2. The initial HU value (75.00) decreased throughout the storage time (p < 0.05). Still, this reduces the progressed in much slower rate in eggs coated with mineral oil than for non-coated, “boomi” wax coated and “dawul kurundu” wax coated eggs. Ovomucin proteolysis, cleavage of disulfide bridges, or by the interaction between α and β ovomucins may all contribute to a decrease in HU value [21]. Compared with non-coated eggs, eggs coated with “boomi” wax, and “dawul kurundu” wax had significantly higher HU during the storage period at room temperature (p < 0.05). According to the HU eggs are divided into 4 grades: AA (> 72), A (72–60), B (59–31), and C (< 30) [7]. Grades of non-coated and “boomi” wax coated, eggs were changed AA to B within 4 weeks whereas in MOs remain in AA.
Albumen pH is also used as an indicator of the quality of eggs egg white [6]. An egg contains of dissolved carbon dioxide (CO2) in the carbonate form (albumen) [10]. If the CO2 and moisture in the albumen evaporates through the pores, more air can enter from the shell. Albumen has a pH of 7.6–8 at first, but when an egg stored in room temperature, CO2 is evaporated through the egg shell pores, increasing the pH rises to 8.9–9.4. Due to the reduction of CO2 gases, pH of the egg white increase and structural changes take place in the egg white and result in a thinning of it [22]. Coated eggs had significantly low pH than uncoated eggs after 7 days of storage (p < 0.05; Fig. 3). pH of the albumin primarily measures the freshness of the egg. Accordingly, coating the eggshell reduce the CO2 infusion through the egg shell. Thus, these coatings act as barrier and help to minimize the diffusion of gases through the shell.
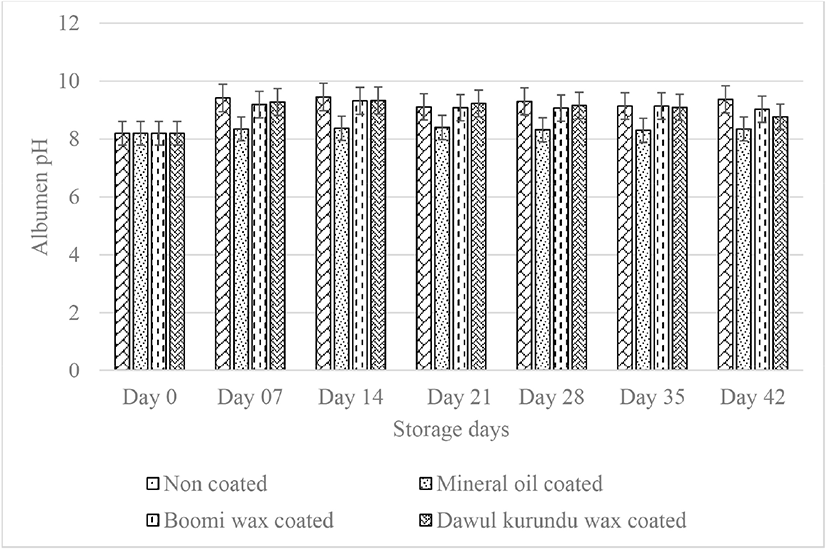
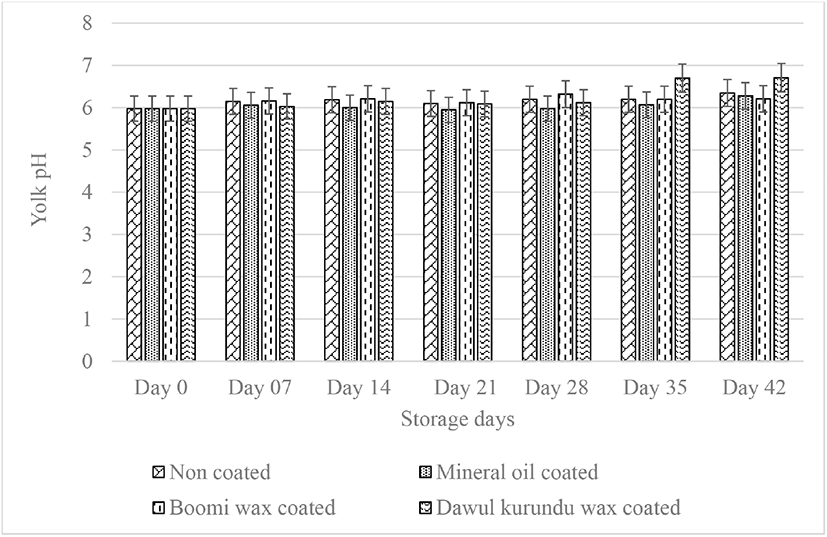
Yolk pH was also increased by storage time. The yolk pH in freshly laid eggs is generally about 6.0, but during storage of eggs, the pH gradually increases to 6.5 [23]. After six weeks of storage initial yolk pH value (5.98) of noncoated, “boomi” wax, “dawul kurundu” wax and mineral oil were increased to 6.35, 6.21, 6.71, and 6.28 respectively. No significant difference was observed in yolk pH in all treatments up to week 4 (p > 0.05; Fig. 4). With the storage, albumen pH increased due to loosing of CO2 and water from the egg white leading to increased pH of the yolk as well [10]. Accordingly, coating of the eggshell declines CO2 infusion. The coatings act as barrier and help diffuse gases less rapidly through the shell.
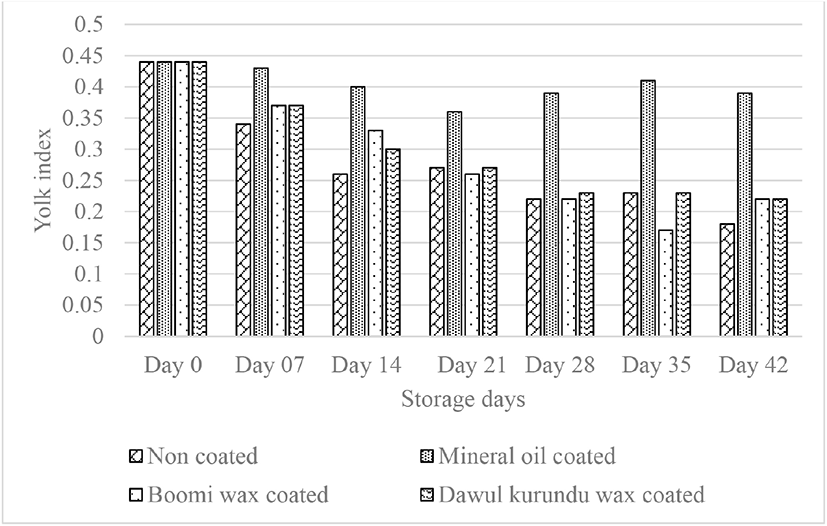
With storage times, the yolk index values generally decreased. Yolk index reduce as a result of a with the weakening of the vitelline membrane, reducing the total solid and liquefaction of the yolk mainly caused by the osmotic diffusion of water from the egg white [6]. Yolk index of non-coated and coated eggs reduced significantly (p < 0.05) with increased storage periods. After storing eggs for six weeks, initial yolk index value (0.44) of noncoated, “boomi” wax, “dawul kurundu” wax and mineral oil were decreased to 0.18, 0.22, 0.22, and 0.39 respectively (Fig. 5). These results indicated that, mineral oil coating has enhancement effect in maintaining yolk quality during storage.
The air cell is situated at the blunt end between the shell membrane and the egg membrane. The egg shell is more porous at this end and therefore air will enter here as the egg contents shrink due to cooling down after laying. During storage the air cell gradually increases in sizes as water evaporates from the egg contents [24]. In this study, the air cell height of all eggs increased with increased storage time during 6 weeks at room temperature (Table 1). After 6 weeks of storage, all coated eggs had significantly (p < 0.05) lesser air cell height than non-coated eggs.
Albumin is a class of simple, water‐soluble proteins that are found in egg white. Albumin is known to have a secondary structure that includes alpha‐helices, parallel beta sheets, antiparallel beta sheets, and random coils. The secondary structure of albumin is sensitive to environmental changes, including changes in pH. Addition of an acid or a base to an albumin solution changes the number and the distribution of the charges on the protein [25]. In the yolk spectrum, bands seemed at the 2,900 and 2,850 cm–1 can be related to the widening vibrations of the C-H group in lipid molecules. The band appaired at the region of 1,750 cm–1 can be linked with widening vibrations of the C = O group of saturated aliphatic esters. In the areas of 1,650 and 1,550 cm–1, there are two amides’ bands (respectively: amide I and II) associated with vibrations of peptide bonds of proteins [26]. Through the storage period (6 weeks), there was no significant difference in absorption units in-between 1,600–1,700 cm−1 wave numbers in all coated and non-coated eggs (Fig. 6). Thus, there were no significant difference (p < 0.05) in secondary structure of egg protein reveals that no chemical structure changes occur due to the application of these coating materials.